What is near-infrared spectroscopy?
Infrared radiation (IR) was first detected in 1800 by Friedrich Wilhelm Herschel who wanted to know if there was a specific color of light that could be related to the warmth of sunlight. Herschel used a prism to separate the colors of light and held a thermometer as a detector to the different rainbow or spectral colors. He found the maximum amount of heat beyond the red end of the visible spectrum. Since there was no visible light, Herschel surmised that there must be an invisible form of light beyond the red end of the visible spectrum. This radiation, which was invisible to him, he called infrared radiation (IR).

Today, we know that infrared radiation can be divided even further, as it is composed of several so-called spectral ranges. To make the various spectral ranges usable, different radiation sources and optical components are built into a spectrometer.
One of the most versatile and useful types of IR spectrometers is the near-infrared (NIR) spectrometer, which covers wavelengths just beyond the visible, i.e., from 780 nm to 2500 nm.

The theory of molecular spectroscopy:
Spectroscopy is an analytical technique that uses the interaction of optical energy with a sample to analyse its composition.
The captured data is displayed in a spectrum, a graphical representation of detected light intensity versus the wavelength of the light.
In IR spectroscopy, molecules absorb certain frequencies of optical energy (photons) that transform the molecules from their "ground state" to "excited states"; these produce oscillations and rotations of the molecular bonds.
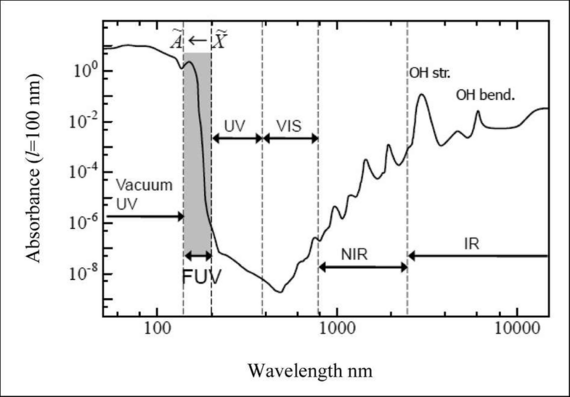
Typically, NIR spectra show broader bands arising from the overlapping harmonics and combination vibrations that occur in more complex molecules. Bonds that can usually be "seen" in NIR spectra are H-C, H-O, H-S and H-N. Organic materials often contain these molecular H-bonds, and therefore biological and organic materials are often analyzed with NIR spectroscopy.

General spectrometer design and optical components
The purpose of a spectrometer is to measure the "amount of light" at different wavelengths separately to selectively see "every color of the rainbow". Every spectrometer consists of some general optical components: Input and collimating optics (light source), diffraction element (grating) and focusing optics to direct the spectrum to the detector (array).
Light source: The light source emits the specific infrared radiation, and different sources are used for the different spectral ranges. For NIR spectroscopy, a halogen lamp is used.
Diffraction element/grating: A type of prism is used to split the light emitted by the light source into the different spectral ranges. The Polytec NIR spectrometers use a grating which is an optical element that diffracts (splits) electromagnetic radiation according to the energy difference. A common type is a reflective surface with a regular array of lines/grooves etched into the surface. The number of lines, profile and depth determine the spectral range, efficiency, resolution and overall performance. For a line grating, lines are often cut into a glass substrate with a reflective coating using a diamond-tipped tool.
The second important type of grating is the holographic volume phase grating. Holographic gratings are produced by a photolithographic process, usually in a matrix material (dichromated gelatin) sandwiched between transparent covers (glass). Diffraction occurs due to a periodic change in the refractive index at constant thickness.
Detector/photodiode array: In the spectrometer, a photodiode array is used as the detector unit to measure light intensity. A photodiode array is a semiconductor component. The detector element consists of a group of linearly arranged photodiodes as well as a supply and readout circuit. The semiconductor material used for these diodes depends on the spectral sensitivity for the required wavelength range. For the near-infrared spectrum and the spectral range of 900-2500 nm, InGaAs (indium gallium arsenide) or PbS (lead sulfide) is used.
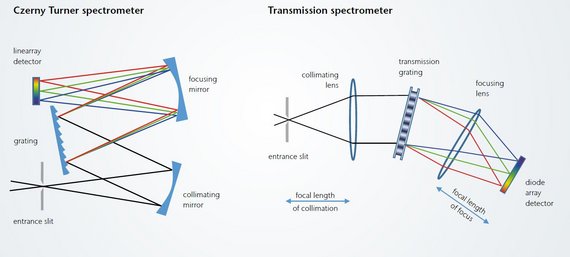
Spectrometer constructions: CzernyTurner (CZ) and transmission spectrograph (TR)
Two very commonly used spectrometer designs are the Czerny-Turner spectrograph, which is usually based on a reflective grating and mirrors, and the transmission spectrograph.
The biggest problems with CZ spectrographs are stray light due to grating imperfections, the lower throughput of the system due to the reflective grating and the lower reflectivity of mirrors compared to the transmission of lenses. In addition, mirror-based systems are more difficult to compensate for optical errors such as chromatic aberration or astigmatism.
Advantages of transmission design:
The main advantage of a transmission grating-based spectrometer is higher throughput (compared to reflectance spectrometers). The greatest contribution to this is the higher diffraction efficiency of volume phase holographic gratings (VPH) which results from the higher regularity of VPH gratings and the possibility to apply anti-reflective coatings to the covers of VPH gratings. Reflective gratings (and the mirrors used in a CZ spectrograph) have a lower throughput due to surface losses in the reflection process. When designing VPH gratings, there are also more parameters to optimize the grating for the intended wavelength range. The relationship between efficiency and wavelength is quite homogeneous for VPH gratings, while reflection gratings are optimized for a specific wavelength and their efficiency decreases significantly outside it.